Pulsetto Reviews
We test Pulsetto, a hands-free vagus nerve stimulator, to see how it holds up against the science and the competitive field.

Photo by Innerbody Research
You’ve likely suffered a headache or a poor night’s sleep at some point in your life. Probably, the demands of work or school have overwhelmed you, too. It wouldn’t be unimaginable if all such problems were to fall on you on the same day.
Were that to happen, a device like Pulsetto might be able to help. Emitting high-frequency electrical waveforms at a clinically studied frequency, Pulsetto can alter the signals in your vagus nerve to impart a range of potential benefits for pain, mood, and more. In our Pulsetto review, we examine how it works, measure it against competitors, and report our testers’ experiences with the product.
Our Findings
Pulsetto represents a relatively low-cost entry point in the at-home transcutaneous vagus nerve stimulator (tVNS) space. Even with ongoing expenses like conductivity gel and (if you’d like) a premium app subscription, the total cost of ownership after 1-2 years is lower than that of a competitor like Truvaga Plus. Our testers saw decent outcomes in subjective stress and sleep quality markers. At the same time, Pulsetto boasts less scientific support for its exact operating parameters than Truvaga does — currently, the only research that specifically favors Pulsetto comes from thesis candidates who received funding from Pulsetto. And though our testers felt therapeutic effects, they agreed that those benefits with Pulsetto were milder compared to Truvaga. So in a nutshell, this seems to represent an opportunity to achieve more modest improvements at a lower price.
Pros
- Uses an operating frequency similar to an FDA-cleared vagus nerve stimulator
- Decent therapeutic outcomes among our testers
- Lower up-front cost and total cost of ownership compared to competitors
- Shorter sessions compared to some competitors
- Hands-free operation
- Adjustable timer allows for finer control over session lengths
- A large number of features in the connected app
- Decent battery life
- HSA/FSA eligible
- 30-day money-back guarantee
Cons
- No unaffiliated or peer-reviewed research behind Pulsetto’s exact operating parameters
- Weaker therapeutic effects compared to a leading competitor, according to our testers
- Device may be too large for some necks
- Premium app features probably aren’t worth the extra expense
- Requires application of conductivity gel, which can be a little messy
- Limited customer support channels
- Shipping delays aren’t uncommon
Purchase options
The Pulsetto device, electrolyte gel, and premium app access can all be purchased through the official Pulsetto website. The device ships for free, but the gel doesn’t. The device also comes with a 30-day trial of the premium app, a 30-day money-back guarantee, and a two-year warranty. Please note that shipping delays aren’t uncommon, as our testers and numerous online reviewers have attested.
You can also buy the Pulsetto device (though not the gel or app subscription) through the company’s Amazon storefront for a negligibly lower price. The cost of shipping will negate the price difference unless you have a Prime membership. Therefore, we recommend purchasing directly from Pulsetto unless you have an urgent need for the device and can’t risk the potential shipping delay.
Jump to:
Why you should trust us
Over the past two decades, Innerbody Research has helped tens of millions of readers make more informed decisions about staying healthy and living healthier lifestyles.
Our Pulsetto review builds off of the research we started with a competitor, Truvaga. As we write this, our research hours into transcutaneous vagus nerve stimulation exceed 130 hours. That includes the time we’ve put into hands-on testing and company correspondence, not just the close readings of scientific literature. With our holistic approach to understanding the therapeutic space Pulsetto occupies, we can present the most complete picture possible of its merits and shortcomings.
Additionally, like all health-related content on this website, this guide was thoroughly vetted by one or more members of our Medical Review Board for accuracy and will continue to be monitored for updates by our editorial team.
How we evaluated Pulsetto
We based our overall rating of Pulsetto on five factors that are likely to influence consumer decisions:
- Effectiveness: Does Pulsetto do what it says it does? What science is there to support it?
- Safety: What health risks, if any, does it pose to a general audience? What about specific populations?
- Cost: Vagus nerve stimulators are often expensive. How does Pulsetto compare in that regard?
- User experience: What’s it like to use the device and its connected app? Are there any issues I might encounter?
- Customer experience: How easy or difficult is it to deal with Pulsetto’s customer support team? Should you expect any obstacles?
Let’s see how Pulsetto performed in each category.
Effectiveness
It has a lot to do with our testers’ positive experiences that Pulsetto’s effectiveness rating rises on tiptoe above the midpoint. In their week of daily multi-session use, they felt noticeable relief from frequent ailments such as sleeplessness, stress, and stress-related headache. For them, Pulsetto and the associated programs in the connected app did what they advertised, albeit not to the same degree as they had with a competitor, Truvaga Plus.
The upward-leaning score also has to do with the similarities between Pulsetto’s operating parameters and those of an FDA-cleared device called gammaCore. The well-tested, prescription-only gammaCore emits a pulse frequency of 5,000Hz with a pulse-repetition frequency of 25Hz, which studies have found to be effective in treating stress-related psychiatric disorders in two-minute sessions, as well as in increasing mental responsivity.1 2
But similar isn’t identical. Pulsetto’s pulse frequencies deviate from 5,000Hz by several hundred hertz in either direction, depending on the chosen treatment program, and such deviation can mean a significant difference in efficacy. Also, as it stands, the only studies that support Pulsetto’s exact operating parameters are thesis-level research funded by Pulsetto itself.
With all that considered, along with our testers’ preference for a competitor, we think Pulsetto is decently effective but not superlatively so.
Safety
Vagus nerve stimulators are generally safe and well tolerated when used within common operating parameters,3 and because Pulsetto’s parameters hew pretty close to gammaCore’s, we’d say they fit that mold. Moreover, Pulsetto is certified by the Federal Communications Commission, meaning that its radio frequency emissions are within safe levels.4 5 In terms of your exposure to electromagnetic radiation, using Pulsetto ought to be no more hazardous than operating your cell phone.
Having said that, we acknowledge that Pulsetto’s maximum output of 30V is greater than gammaCore’s, and that may increase the risk of side effects both minor and major — for example, headache, dizziness, heart palpitations, and skin reactions6 — and the dearth of research into its exact operating parameters leaves specific safety questions unanswered.
Again, though, we can point to our testers as evidence that Pulsetto is more safe than not. One of them discovered only too late that he was contraindicated for use because of a metallic implant in his jaw, yet he experienced no ill effects as a result.
Cost
Compared to other products in a relatively high-cost space, Pulsetto represents a bargain. Here’s a table to illustrate:
| Pulsetto | Truvaga Plus | VeRelief Prime | Xen | |
|---|---|---|---|---|
| Device (lowest $) | $269 | $499 | $249 | $449 |
| Conductivity medium per year ($) | $81-$102 | $45 | $200 | $10 maybe |
| Other ongoing purchases per year | $0-$129 (optional) | NA | NA | NA |
| Total cost of ownership, 1 year | $350-$500 | $544 | $449 | $549 approx. |
(Note: The conductivity medium refers to the electrolyte gel or spray sold through the company website. You don’t have to buy these products through the website or even the specific brands they sell. In fact, it’s much less expensive to buy them elsewhere. But we’ve used the brand-specific products in the table as a way to standardize pricing as much as possible. You’ll see more nuanced price breakdowns later in this review.)
You see that Pulsetto’s total cost of ownership after one year is a pretty wide range. You reach the top end of the range only if you purchase the premium app on a month-to-month basis (not recommended for everyone) and are over-generous with your use of electrolyte gel. Even then, the cost is less than Truvaga Plus and Xen after one year. And on the low end, Pulsetto’s one-year total cost of ownership beats all the other competitors by a wide margin.
User experience
Pulsetto’s size-adjustable, hands-free form factor visibly sets it apart from competitors Truvaga Plus and VeRelief Prime, which the user must manually hold to their neck for 2-10 minutes to operate. Most Pulsetto sessions are ten minutes long but can be adjusted to be as brief as four, and our testers found it much less intrusive to their schedules to wear Pulsetto for longer sessions than to pause all other activities to complete a shorter session with Truvaga or VeRelief. If you’re so inclined, you could complete your Pulsetto sessions while doing other activities, like working out or preparing dinner, as one of our testers did.
Unfortunately, hands-free operation, even with a size-adjustable device, is more of a burden than a boon for people with slender necks. One of our testers, for example, has a 14in neck circumference, and the device’s electrodes extended away from skin contact by at least a finger’s breadth. She had to move the device backward so that the electrodes clung to her neck by friction, which forced her to limit her movement during sessions.
The connected app, at least, is intuitively designed. Without reading a manual, you could probably navigate your way to and through a session without a hitch. Most of the programs’ names clearly indicate what they’re supposed to do, and the session interface clearly marks options for increasing intensity and adjusting the time frame.
But an app interface isn’t ideal in all situations. Let’s take, for example, a headache, one of the ailments that vagus nerve stimulation can treat. Imagine your head explodes with pain with every heartbeat and your phone’s Bluetooth won’t connect to your Pulsetto device. How swiftly do you think you could troubleshoot the connection problem so that you can find symptom relief?
There’s also the matter of the paid version of the Pulsetto app — called Pulsetto Premium. It’s through this version that you get the treatment programs for Head Pain, Inflammation, and Gut Health, as well as supplemental features for meditations, breathing exercises, and affirmations. The three extra treatment programs could be useful, yes, but the supplemental features seem as though their purpose is to justify the existence of a paid tier. Aside from our testers having had no use for them, similar features are widely available through other (free) apps and platforms. So, depending on what ailments you normally experience, you might be better off sticking with the basic app and using the Stress or Pain program to treat things like headache and gastrointestinal pain.
Customer experience
The customer experience with Pulsetto is an evenly distributed mix of good and not-so-good.
On the good side, there’s a 30-day satisfaction guarantee and a decently responsive support team. True, the only way to contact Pulsetto is by email, the slowest of the common communication channels for customer support, but the company’s reps have been good about responding to our queries within 1-2 days. Seeing as many companies we’ve reviewed have taken up to a week just to acknowledge our emails, we commend Pulsetto’s attentive turnaround. We especially appreciated the quick refund we received — on a weekend, no less — on an erroneous charge for Pulsetto Premium before our 30-day trial was up.
On the not-so-good side, the very reason for the erroneous charge seems to be a common issue among Pulsetto’s customers. What happened was that our order had a 12-day delay between purchase and shipment, and the company started our premium app trial on the date of purchase. In effect, we had only half the time we were promised to try the premium app before being hit with an unexpected charge. Again, an email quickly resolved the issue, but we think the issue itself should never have arisen. Bear in mind that Pulsetto’s Trustpilot page displays many instances of similar, if not longer, delays, so you may face the same problem we did.
The basics of transcutaneous vagus nerve stimulation
Pulsetto belongs to a therapeutic space called transcutaneous vagus nerve stimulation (tVNS), a form of electrical pulse treatment. The pulses are delivered not by implant but via electrodes placed on the skin, so it’s fully noninvasive. The electrical pulses, aided by a conductivity medium such as electrolyte gel, travel through your skin and into your vagus nerve.
The vagus nerve is the longest nerve of the autonomic nervous system, starting at the lower brainstem and extending to the digestive tract. Along the way, it passes through or connects to the neck, heart, and lungs.7 The nerve’s placement in the body allows it to be stimulated auricularly (by the ear canal) or cervically (by the neck).
By stimulating the vagus nerve, tVNS can potentially alter neuronal signals that affect multiple regions of the brain and the body, imparting relief for conditions such as:8 9
- Epilepsy
- Mood disorders
- Chronic pain
- Headache, including cluster headache
- Cardiovascular disorders10
- Stress1
- Sleep disorders11
- Cognitive function and attention12 13
The ability of tVNS to provide these health benefits may depend on the operating frequency used. Most clinical studies have used a 25Hz frequency, which appears to improve biomarkers related to stress, but others have seen benefits from higher frequencies (e.g., 100Hz leading to the greatest decrease in heart rate, suggesting a more pronounced effect on stress) as well as lower ones (e.g., 20Hz for improved cardiovascular health).14 15
What is Pulsetto?

Photo by Innerbody Research
Pulsetto is a hands-free device similar to headphones, except the ends clamp onto the sides of your neck rather than your head. At each end are two electrodes oriented vertically. Before each session, you’re to apply a small amount of the included electrolyte gel either directly to the electrodes or to the treatment sites on your skin (i.e., the areas that align with your carotid pulse).
Insider Tip: A couple of notes about the gel. Pulsetto recommends using a pea-sized amount of it for each session, but our testers experienced decent results with maybe half as much gel spread across all four electrodes. They also preferred applying the gel directly to the electrodes because it gave them a clearer sense of how much to use. Any excess they rubbed onto their necks.
Pulsetto is an app-connected device. As with competitors Truvaga Plus and Xen, the app is the hub through which all sessions take place. You activate your phone’s Bluetooth, connect Pulsetto to your phone, and then select a treatment program through the app interface.
There are two versions of the Pulsetto app:
- The basic (free) version has programs for Stress, Anxiety, Sleep, Burnout, and Pain.
- The premium (paid) version includes programs for Head Pain, Inflammation, and Gut Health, as well as meditation programs, breathing exercises, affirmations, and a test to evaluate your neuromuscular function.
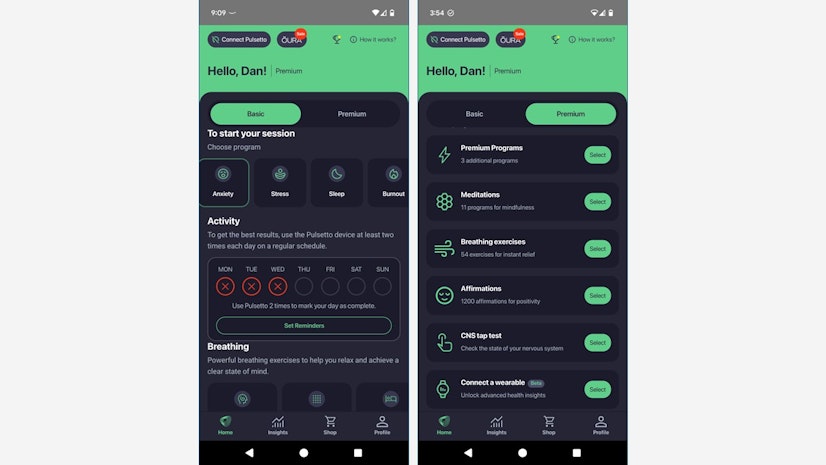
Photo by Innerbody Research
Each treatment program emits a specific pulse frequency, each at a 25Hz pulse-repetition frequency (PRF) — a common frequency used in clinical research.14 The Stress program, for example, produces a 4,500Hz pulse frequency. In relation to the PRF, that means the device emits 4,500 electrical oscillations in every pulse, with 25 pulses per second. The pulse frequency ranges up to 5,200Hz depending on the program you choose.
Some tVNS studies have used a similar but not identical electrical pattern — a 5,000Hz pulse frequency with a 25Hz PRF— including a 2017 study on transcutaneous vagal access via the neck, a 2020 study on treating stress-related psychiatric disorders, and a 2022 study that demonstrated increased mental responsivity in subjects who received noninvasive vagus nerve stimulation.16 1 2 A prescription product called gammaCore, currently the only FDA-cleared and CE-marked tVNS device, also uses a 5,000/25Hz electrical pattern,17 18 19 as does our favorite nonprescription device, Truvaga Plus.
Pulsetto studies
While research supports using the 5,000/25Hz stimulation pattern, the only sources that vouch for Pulsetto’s exact operating parameters are those whose funding came from Pulsetto itself. What’s more, none of them appear to be published in peer-reviewed journals, and two of them are explicitly master’s theses. Still, let’s see how they collected their data and what their findings might mean:
Pulsetto’s effects on stress and heart rate variability
For a 2024 master’s thesis submitted to Erasmus University in Rotterdam, Netherlands, the researcher organized two experiments with wildly different participant parameters: the first with eight atrial fibrillation patients, the second with 40 healthy people. Subjects in each experiment underwent ten-minute sessions with Pulsetto; all used the Stress program, and subjects in the second experiment also used the Burnout and Pain programs. Cardiac markers were measured before and after the sessions.20
According to the paper, subjects who underwent Pulsetto stimulation experienced “significant” decreases in heart rate (suggesting reduced stress levels)21 as well as increases in heart rate variability (suggesting better cardiovascular fitness and stress resilience).22 The sham/placebo group exhibited none of these changes.
Pulsetto’s effects on inflammation
In a pilot study published on the research repository Zenodo in 2025, the author describes a clinical trial that assessed Pulsetto stimulation on seven patients with a chronic inflammatory condition known as ankylosing spondylitis. The participants used Pulsetto twice daily and responded to a self-report questionnaire every day. The results of the questionnaire were that 82% of participants (five people) attested to decreased morning stiffness, and the average scores for joint pain, range of motion, quality of life, concentration, and sleep improved in the majority.23
It’s worth noting that this study seems not to have been placebo-controlled, so participants could have believed they were feeling better only because they were receiving treatment.
Pulsetto’s effects on migraine
A paper published in 2025 relates the outcomes of 20 participants with frequent migraines who underwent Pulsetto treatment for six weeks. Their measures were taken via self-report questionnaires. By the end of the study, migraine frequency had decreased by 40.35%, pain days by 27.66%, and pain intensity by 42.46%.24
The limitations of these studies are apparent: each had a small sample size, and two of them relied entirely on self-reported measures. Thus, they’re less proof of Pulsetto’s efficacy than of the dearth of research relating to it.
On the other hand, their findings more or less correspond to those of our own testers, who used Pulsetto multiple times daily for a week. We discuss our testers’ impressions in a later section, “Our Pulsetto user experience.”
Is Pulsetto safe?
A systematic review published in 2018 concluded that tVNS devices operating within common parameters are generally “safe and well tolerated.”3 Seeing as Pulsetto’s operating frequencies are mostly similar to those of gammaCore, an FDA-cleared and CE-marked product, we can consider them to be “within common parameters.”
We can also point to Pulsetto’s Federal Communications Commission (FCC) certification as further evidence of its safety. Reports from June 2022 show that the Pulsetto device passed all of its tests for electronic emissions and was granted FCC approval.4 In other words, its radio frequency emissions are within generally non-hazardous limits for its class, much like a cell phone.5
That being said, Pulsetto has a higher maximum output than gammaCore — 30V as opposed to 24V — so it may pose a slightly higher risk of side effects seen in research, such as:3 6
- Headache
- Dizziness
- Nausea
- Voice alteration
- Hoarseness
- Coughing
- Tingling
- Shortness of breath
The 2018 systematic review we mentioned also identified three serious adverse events that could “possibly or probably” be attributed to tVNS treatment: heart palpitations, acute vertigo, and localized skin reactions.3
Who is Pulsetto for?
Because of the diverse treatment potential of tVNS, Pulsetto may be a suitable at-home therapy if you experience:
- Stress or anxiety
- Problems focusing
- Difficulty sleeping
- Chronic pain or headache
- Inflammation
- Gastrointestinal distress
That isn’t to say that Pulsetto can resolve these health problems absolutely, as there may be underlying causes at play, but it may provide some degree of relief so that you can manage your symptoms.
Who is it not for?
Pulsetto and other tVNS devices are contraindicated in people who:25
- Have only one vagus nerve
- Have a cardiovascular condition such as arrhythmias
- Have an abnormally functioning autonomic nervous system
- Have a chronic lung disorder such as asthma or shortness of breath
- Have preexisting hoarseness
- Are prone to ulcers
- Are prone to fainting
- Currently receive other forms of electrical stimulation
In addition to the above, Pulsetto warns that its device is not suitable for people with an implantable medical device (e.g., pacemaker, hearing aid implant), a metallic implant near the neck (e.g., stent, plate, or screw), a history of cancer or a head condition (e.g., brain tumor, aneurysm, head trauma), or an abnormal cervical anatomy, as well as children and pregnant women.
Although it’s true that some studies have determined tVNS devices to be safe and effective for use during pregnancy, Pulsetto’s disclaimer holds merit given the uncertainty surrounding the risk of radio frequency exposure during gestation.26 27 So if you’re pregnant and wish to use Pulsetto, you should consult with your physician to ensure that it would be safe for you and the developing fetus.
How much does Pulsetto cost?
Pricing with Pulsetto, as with many leading tVNS products, is complex. There are multiple factors that contribute to the total cost of ownership (TCO):
The device
The Pulsetto device costs $269, with free shipping when purchased through the official website. Pulsetto markets this price as a discount of $200, although we’ve only ever seen the device sold at the “discounted” price. It costs around as much on the Pulsetto Amazon storefront ($267 at this writing, but free shipping only for Amazon Prime members).
With your device, you get one bottle of conductivity gel, a one-month trial of the Pulsetto Premium app, and a two-year warranty.
Conductivity gel
You’ll need to periodically replenish your supply of conductivity gel. The device comes with one bottle. Pulsetto says it should last at least one month with consistent use, but that depends on how much you apply per session. Our testers, after a week of daily use, 2-3 times per day, went through only one-fifth of the provided bottle. If you use Pulsetto more sparingly, you could feasibly stretch a bottle for 2-3 months.
A re-up of four fresh bottles is $50.99 as a one-time purchase or $40.79 on subscription. The four-bottle supply is supposed to be good for 4-6 months, but (again) your mileage may vary. At any rate, you should account for an additional $81-$102 for enough gel to last the year, raising your first-year TCO to around $350-$371.
But (and this is important) those prices are higher than necessary. You can use any conductivity gel you want and purchase it from anywhere you want. Even if you use the same brand of gel that Pulsetto sells (Signa Gel by Parker Labs), you can buy it for as little as $5.49 for a half-year’s supply. More realistically, then, conductivity gel will add just around $11 to your first-year expense, amounting to a TCO of approximately $280.
Pulsetto Premium subscription
If you want continued access to Pulsetto Premium, which unlocks most of the app’s features, you’ll have to pay $129 for a year’s subscription. Consequently, your first-year TCO then increases to around $409-$500.
So the first-year expenditure for Pulsetto is around $280 at the low end (with conservative gel use from a third party and sans premium app) and $500 at the high end. In terms of daily use, the per-diem cost equals about $0.77-$1.37. Not bad. Many of the supplements we review have higher per-day use costs.
And compared to other tVNS devices, Pulsetto’s value is excellent. Take Truvaga Plus, for example, which costs $499 for the device alone. Or compare it to Xen or VeRelief Prime, each of which costs around $449 after one year. Financially, Pulsetto occupies probably the most comfortable range for most people, as it offers both a low barrier to entry and a low cost of continued use.
Also, Pulsetto may be HSA/FSA eligible and comes with a two-year warranty. None of its competitors have as long a warranty, and only Truvaga is explicitly HSA/FSA eligible.
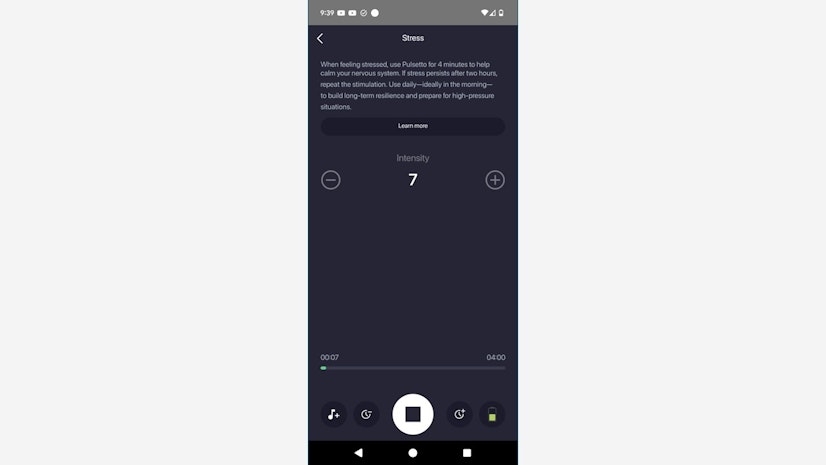
Photo by Innerbody Research
Pulsetto’s money-back guarantee
Like Xen and Truvaga, Pulsetto has a 30-day money-back guarantee that starts from the date of delivery. That gives you a month to decide whether Pulsetto works for you. If it doesn’t, and you want a refund, you just complete the form linked on the Refund Policy page of the company’s website. Within 24 hours, the customer support team should email you with confirmation, your authorization number, and instructions for return. Thereafter, you have 21 days from the receipt of the email to get your device, in its original box along with all included accessories, in transit back to Pulsetto. So, altogether, you effectively have a 51-day trial.
Our Pulsetto user experience
Along with our purchasing Pulsetto and interacting with its customer support team, we had our testers use the Pulsetto device multiple times a day for a week straight. In the following sections, we discuss personal and subjective factors such as our purchasing experience, our feelings about the company’s support quality, our opinions of the Pulsetto device and app, and our testers’ therapeutic responses to Pulsetto. (Please keep in mind that our experiences don’t necessarily reflect others’, and your experience may be very different.)
Buying the Pulsetto device
Purchasing Pulsetto posed no problems, but shipping it did. Two days after we completed our purchase, we received an email from the company notifying us about an extended shipping time frame of up to ten days “due to high demand and courier delays.” The actual time frame was longer, and our order didn’t ship out until 12 days post-purchase. A look through Pulsetto’s Trustpilot page suggests that such delays are common, with some customers reporting that it took weeks before their orders arrived.
Fortunately, Pulsetto’s money-back guarantee doesn’t begin until your order arrives, so you’ll have the full 30 days to test the device even if you experience a delay. The same should go for the 30-day trial of Pulsetto Premium, but you may have to email customer support about that (which we did).
Pulsetto’s customer support
The only way to contact Pulsetto’s support team is by email, so while our interactions with them were generally positive, they weren’t as expedient as we would have liked. In our initial exchanges, in which we asked about Pulsetto’s exact operating parameters, we received our first responses on the same day, but subsequent messages took several days to arrive. All told, it took a full week to resolve the question of what frequencies Pulsetto used.
To the team’s credit, they were relatively responsive considering the nature of the communication channel. While their responses weren’t as speedy as the ones we got from competitor Xen, some companies in the space take a week to respond to just the initial message. We wish, though, that Pulsetto would add a phone line or, like Truvaga, a live chat feature on its website.
At any rate, a better experience came later, when we were charged for a Pulsetto Premium subscription well before our 30-day trial had ended. The issue was that the company had started our trial upon the date of purchase, not the date of arrival. We immediately emailed the support team about this issue, but seeing as it was a Saturday, we didn’t expect a response until Monday. Yet the response came just a few hours later — first as a notification of a refund, and then as an explanation of the company’s error. The team then reset our premium app trial so that it aligned with the 30-day window starting from the date of arrival.
Using the device
Our testers — we’ll call them “Ben” and “Jo” — had differing opinions about the Pulsetto device itself.
Ben found that the electrodes rested comfortably against the pertinent areas of his neck; only at first did it feel like an overly tight barber’s cape, but that feeling resolved on its own at around the fourth use.
Jo, however, has a more slender neck that Pulsetto poorly accommodated. She had to position it more rearward so that the “headband” portion of the device rested on her upper trapezius muscles and the electrodes held on by friction rather than organic placement.

Photo by Innerbody Research
The fit difference led to no disparities in therapeutic benefit as long as Jo didn’t disrupt the device’s positioning during her sessions. But that means that she couldn’t use Pulsetto simultaneously with other activities, like working out or preparing dinner, the way Ben could.
Insider Tip: Wipe down the electrodes and your neck after use. Otherwise, the trace electrolyte gel that remains on either surface will dry into a film that resembles a post-sunburn peel.
Despite using the device multiple times every day, our testers never needed to charge it (they never even unwrapped the charging cable included in the box). Yet the battery life stood at around two-thirds full by the end of the testing week.
The pulsetto app
Ben and Jo found the Pulsetto app easy to use and feature-packed, but they personally saw little value in most of its features.
The interface is split into two tabs: Basic and Premium. The Basic tab has the programs for Stress, Anxiety, Sleep, Burnout, and Pain, and that’s where our testers spent most of their time. The Premium side includes the Head Pain, Inflammation, and Gut Health programs, as well as meditations, breathing exercises, affirmations, and a neurological function test. Our testers derived no benefit from those latter, non-treatment features and didn’t think they did anything to justify the subscription price.
Session times vary depending on the program. The Sleep program, for example, lasts ten minutes by default, whereas the Stress program lasts six. You can also adjust each program’s time frame within a four-minute range — two minutes more or two minutes less — as well as the intensity level from 1 to 9. If you wish, you can customize your session with a file from Pulsetto’s music library, each of which is something that a new-age massage therapist might play in their studio.
(By the way, your device’s battery life is displayed in the lower right-hand corner of the session screen, which is helpful even though our testers didn’t need to recharge their device during the testing week.)
Our testers’ therapeutic responses to Pulsetto
Ben and Jo centered their self-guided treatments on health issues they normally experience: sleeplessness and stress. We contextualize and summarize their impressions below:
Pulsetto for sleep
The Sleep program, part of the basic app plan, is a ten-minute session to be completed 30 minutes before bed. Ben undertook a session each night of the testing week, experimenting with intensity levels between 6 (one level above the median) and 9 (the maximum). He didn’t care for the ten-minute length, much preferring the shorter, two-minute sessions of Truvaga Plus, but he also found that Pulsetto could be more easily integrated into his activities. Usually, by starting his session near the end of a movie or series episode, he could largely forget that electricity was pulsing through his neck. Also — and he didn’t realize this was a contraindication until later — Ben does have a metallic implant in his jaw from a long-ago accident, but he didn’t experience any attendant ill effects.
As for his sleep quality, he reports that he felt drowsier than he normally would on getting into bed, that he could fall asleep faster, and that he would sleep more deeply than usual. On two nights, however, he experienced multiple, albeit brief, sleep interruptions, a possible cause of which could have been anxiety — not a great sign of efficacy for improved heart rate variability.28 29 He noted that such interruptions didn’t occur with Truvaga Plus.
Pulsetto for stress/headache
Jo is an art teacher who plans and coordinates an annual student art show, and her testing coincided with the busiest period of her school year. On multiple evenings, while working after hours, she would experience a growing pain in her forehead — an indication of a tension headache.30 Initially, to treat it, she used the Head Pain program included in Pulsetto’s premium app plan, but it yielded no benefit. But then, figuring that stress was the root of her pain, she switched to the Stress program and did feel some relief. In her estimation, the pain decreased “by about half” each time, which was enough to let her continue with her work without much bother.
Ben also used the Stress program daily. He states that it provided him with brief mental boosts, a claim that aligns with clinical findings on stress’s relationship to executive function.31
Insider Tip: On starting sessions, our testers consistently experienced a four- or five-second delay between hitting the “play” button and feeling the first electrical pulses. We recommend not increasing the intensity until the delay has resolved. Otherwise, you could receive an unpleasantly high stimulation all at once, without the opportunity to ease into your tolerance level.
Alternatives to Pulsetto
Although Pulsetto offers probably the most accommodating price point for effective at-home vagus nerve stimulation, you may prefer a device with more peer-reviewed scientific support behind it. Or maybe you want a tech-free solution instead. In either case, these alternatives may give you what Pulsetto can’t:
Direct competitors
The best nonprescription tVNS device we’ve tried is the app-connected Truvaga Plus. In terms of operating parameters, it’s almost identical to the FDA-cleared gammaCore (made by the same company, in fact). It’s also programmed to have a useful life of at least 30,000 sessions, and our testers claim it has given them the most significant relief from stress, sleeplessness, and brain fog. It’s pricey, though, at $499 for the device and a first-year TCO of $544 after accounting for conductivity medium.
But there are some options that are less expensive than Truvaga Plus, though not as affordable as Pulsetto:
- Truvaga 350: a lower-cost, app-free version of Truvaga Plus. It costs $299 but is also limited to 350 sessions, so it’s not cost-effective for people seeking a long-term solution.
- VeRelief Prime: an app-free device that offers a simpler approach to cervical tVNS than Pulsetto or Truvaga Plus. Rather than a conductivity gel, it uses proprietary gel tips. It costs $399 for the device alone or $249 if you buy it as part of a gel tip subscription. The one-year total cost of ownership ranges from $449 to $511 depending on your choices among various cost variables.
- Xen: an earpiece device that stimulates the auricular branch of the vagus nerve. The device costs $449. The only necessary ongoing purchase is a conductivity medium, and Xen’s support team says you can use something as inexpensive as contact lens solution for that (maybe an extra $10 per year).
Tech-free solutions
A tech-free, albeit precisionless, solution for vagus nerve stimulation is available to most people at most times. Say that you have a cold bottled drink in your hand: you can press it to the side of your neck and potentially lower your stress level via cardiac-vagal activation.32 Or if you have time for exercise, you can enhance your cardiac vagal activity with a quick run or other form of endurance training.33 If nothing else, you can take a few deep breaths to lower your heart rate and affect your autonomic nervous system.34
Pulsetto FAQ
Sources
Innerbody uses only high-quality sources, including peer-reviewed studies, to support the facts within our articles. Read our editorial process to learn more about how we fact-check and keep our content accurate, reliable, and trustworthy.
Bremner, J. D., et al. (2020). Application of noninvasive vagal nerve stimulation to stress-related psychiatric disorders. Journal of Personalized Medicine, 10(3), 119.
Lerman, I., et al. (2022). Non-invasive cervical vagus nerve stimulation effects on reaction time and valence image anticipation response. Brain Stimulation, 15(4), 946-956.
Redgrave, J., et al. (2018). Safety and tolerability of Transcutaneous Vagus Nerve stimulation in humans: A systematic review. Brain Stimulation, 11(6), 1225-1238.
Federal Communications Commission Report. (2022). 2A5T3-BXN-PU-22V001. FCC.
Federal Communications Commission. (n.d.). RF safety FAQ. FCC.
Mandalaneni, K., & Rayi, A. (2023). Vagus nerve stimulator. StatPearls [Internet].
Cleveland Clinic. (2022). Vagus nerve. Cleveland Clinic.
Hilz, M. J. (2022). Transcutaneous vagus nerve stimulation — a brief introduction and overview. Autonomic Neuroscience: Basic & Clinical, 103038.
Cleveland Clinic. (2022). Vagus nerve stimulation (VNS). Cleveland Clinic.
Capilupi, M. J., Kerath, S. M., & Becker, L. B. (2020). Vagus nerve stimulation and the cardiovascular system. Cold Spring Harbor Perspectives in Medicine, 10(2), a034173.
Wu, Y., et al. (2022). Transcutaneous vagus nerve stimulation could improve the effective rate on the quality of sleep in the treatment of primary insomnia: A randomized control trial. Brain Sciences, 12(10), 1296.
Wang, W., et al. (2024). Advances in VNS efficiency and mechanisms of action on cognitive functions. Frontiers in Physiology, 15, 1452490.
Zaehle, T., & Krauel, K. (2021). Transcutaneous vagus nerve stimulation in patients with attention-deficit/hyperactivity disorder: A viable option? Progress in Brain Research, 264, 171-190.
Yokota, H., et al. (2022). Effects of stimulus frequency, intensity, and sex on the autonomic response to transcutaneous vagus nerve stimulation. Brain Sciences, 12(8), 1038.
Jiang, Y., et al. (2020). Non-invasive low-level tragus stimulation in cardiovascular diseases. Arrhythmia & Electrophysiology Review, 9(1), 40-46.
Frangos, E., & Komisaruk, B. R. (2017). Access to vagal projections via cutaneous electrical stimulation of the neck: FMRI evidence in healthy humans. Brain Stimulation, 10(1), 19-27.
BioSpace. (2018). electroCore receives FDA clearance for gammaCore™ (nVNS) for adjunctive use for the preventive treatment of cluster headache in adults. BioSpace.
Mwamburi, M., Liebler, E. J., & Tenaglia, A. T. (2017). Review of non-invasive vagus nerve stimulation (gammaCore): Efficacy, safety, potential impact on comorbidities, and economic burden for episodic and chronic cluster headache. AJMC, 23(17).
Alzheimer’s Drug Discovery Foundation. (2023). Vagal nerve stimulation. ADDF.
Van den Bogaert, F. (2024). The effect of transcutaneous cervical vagus nerve stimulation on the cardiac autonomic nervous system. Erasmus University Rotterdam.
American Heart Association. (2024). Stress and heart health. AHA.
Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258.
Dirsė, V. (2025). Evaluating non-invasive vagus nerve stimulation for pain management in Bechterew disease: A pilot study. Zenodo.
Dirsė, V. (2025). Evaluating the efficacy of bilateral non-invasive vagus nerve stimulation in reducing migraine symptoms: A prospective observational study. Zenodo.
American Association of Neurological Surgeons. (2024). Vagus nerve stimulation. AANS.
Ding, J., et al. (2021). Is vagal-nerve stimulation safe during pregnancy? A mini review. Epilepsy Research, 174, 106671.
Johnson, E. E., et al. (2024). The effects of radiofrequency exposure on adverse female reproductive outcomes: A systematic review of human observational studies with dose-response meta-analysis. Environment International, 190, 108816.
Harvard Health Publishing. (2022). Top 4 reasons why you're not sleeping through the night. Harvard Medical School.
Chalmers, J. A., et al. (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 5, 80.
Mayo Clinic. (2023). Tension headache. Mayo Clinic.
Liu, Q., et al. (2020). Impact of chronic stress on attention control: Evidence from behavioral and event-related potential analyses. Neuroscience Bulletin, 36(11), 1395-1410.
Jungmann, M., et al. (2018). Effects of cold stimulation on cardiac-vagal activation in healthy participants: Randomized controlled trial. JMIR Formative Research, 2(2), e10257.
Gourine, A. V., & Ackland, G. L. (2018). Cardiac vagus and exercise. Physiology, 34(1), 71-80.
Magnon, V., Dutheil, F., & Vallet, G. T. (2021). Benefits from one session of deep and slow breathing on vagal tone and anxiety in young and older adults. Scientific Reports, 11(1), 1-10.
Sabino-Carvalho, J. L., et al. (2018). Non-invasive vagus nerve stimulation acutely improves blood pressure control in a placebo controlled study. The FASEB Journal, 31, Supplement 848.8.


